
[Abstract] Patent applications involving biology and medicine have broad application prospects in production practice, and patent applications involving sequences account for a considerable proportion of such patent applications. The aim of this paper was to analyze the differences between China and Europe in the evaluation criteria for supporting patent applications involving sequences through three cases, with a view to providing a reference for applicants in relevant fields.
[Key words] sequence; supporting; differences between China and Europe
1. Introduction
In recent years, considerable progress has been made in the field of biotechnology. It is clear that patent applications involving biology and medicine are important for protecting future progress. Patent applications involving sequences account for a considerable proportion of such patent applications. In the examination practice of such patent applications, in addition to the issues of novelty, inventiveness and practicability, the issue of whether the claims are supported by the description occupies a considerable proportion.
Paragraph 4, Article 26 of the patent law of the People’s Republic of China stipulates that “the claims shall be supposed by the description and shall define the extent of patent protection sought for in a clear and concise manner", which provides a theoretical basis for the evaluation of whether the claims are supported by the description. Notably, there are many issues concerning whether the claims are supported by the description in patent applications involving biology and medicine. The reason is that there is a high degree of diversity in the sequences, and thus a high degree of unpredictability. Due to the high degree of diversity, the applicant can only verify a limited number of variable forms through a limited number of experiments, and cannot enumerate all variable forms. Therefore, in order to seek protection, the applicant often wants to protect as wide a range as possible when drafting. However, the high diversity will lead to the claims covering a large number of variable forms that have not been verified by experiments. As such, during the examination, whether the claims are supported by the description has always been the focus in the process of patent confirmation.
The aim of this paper was to analyze the differences between China and Europe in evaluation criteria for supporting patent applications involving sequences through the following three cases, with a view to providing a reference for the drafting and stabilizing of patents of biological and pharmaceutical enterprises in China.
2. Case analysis
[Case 1] the difference between China and Europe in the size of sequence modification: PCT international application PCT/NL2015/050377, China National Phase application 201580039023.6 and European phase application EP15731411A
Overview of China National Phase prosecution:
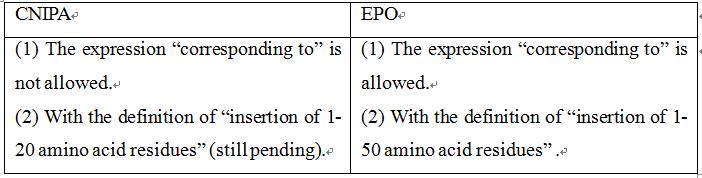
Claim 1 of the application as originally submitted was: "A recombinant protein comprising a Factor Xa polypeptide between the amino acid residue region corresponding to Gly-289 and Asp-320 of SEQ ID NO: 1, preferably between His-311 and Asp-320 has a change in the region of the amino acid residue; wherein said change is the insertion of at least one amino acid residue."As the examiner in the First Office Action issued a rejection based on lack of support, claim 1 was modified to be: "A recombinant mammalian protein comprising a Factor Xa polypeptide between the amino acid residue region corresponding to His-0311 and Asp-320 of SEQ ID NO: 1, of which the altered protein is catalytically active and has a decreased sensitivity to inhibition by a direct coagulation fator Xa inhibitor; and wherein said change is the insertion of 1-50 amino acid residues." The examiner, in the Second Office Action, again issued a rejection based on lack of support. According to this office action, claim 1 was further amended to be "A recombinantprotein comprising a human coagulation Factor Xa polypeptide, said polypeptide having an insertion of 1-20 amino acid residues between His-0311 and Asp-320 of SEQ ID NO: 1, of which the altered protein is catalytically active and has a decreased sensitivity to inhibition by a direct coagulation fator Xa inhibitor.". Later, in the Third Office Action, as the applicant held the technical solution of “polypeptide having an insertion of 1-20 amino acid residues” without making amendments, the examiner rejected the application. The application entered the re-examination stage with the technical solution of “said polypeptide having an insertion of 1-20 amino acid residues”, and a Decision of Re-examination has not been recieved at the present stage.
Overview of European Phase prosecution:
Claim 1 of the application as originally submitted was: “A recombinant protein comprising a coagulation factor Xa polypeptide, said polypeptide having an alteration in a region of amino acid residues corresponding to the region of amino acid residues between Gly-289 and Asp-320, preferably between His-311 and Asp-320 of SEQ ID NO: 1; wherein the alteration is an insertion of at least one amino acid residue.” That is, the claim is the same as claim 1 originally submitted in China. Later, the applicant submitted a modification, and functionally defined the altered protein in claim 1, that is, defining “which altered protein has a decreased sensitivity to inhibition by a direct coagulation factor Xa inhibitor”. With regard to this claim, the European examiner issued a First Office Action, in which the inventiveness of this functional definition was found, but the examiner considered that as there was no upper limit on the size of the insertion, the claim covered a large number of proteins that lost their functions, that is, it covered a wide scope of protection and was not supported by the description. When responding to the First Office Action, the applicant defined the size of the insertion to be “wherein the alteration is an insertion of 1-50 amino acid residues”, thus having a similar scope of protection to the claims submitted in response to the First Office Action in China. Later, the applicant made modifications to overcome further novelty issues, but the modifications were only limited to defining the source species of the protein and did not further reduce the size of the insertion. Finally, the granted claim 1 in Europe was: “A recombinant protein comprising a mammalian coagulation factor Xa polypeptide, said polypeptide having an alteration in the region of amino acid residues corresponding to the region between Gly-289 and Asp-320, preferably between His-311 and Asp-320 of SEQ ID NO: 1; wherein said alteration is an insertion of 1-50 amino acid residues, and wherein said altered protein has a decreased sensitivity to inhibition by a direct Factor Xa inhibitor when compared to said mammalian coagulation factor Xa polypeptide not having said alteration.”
Comparison between the prosecutions in China and in Europe:
Taking a look at the examination opinions made during the prosecution in China, it can be found that the applicant gradually narrowed the scope of the claims, and tried to prove from the mechanism that the technical solution of “polypeptide having an insertion of 1-20 amino acid residues” could be supported by the description. The applicant believed that the application as filed in combination with the prior art could indicate that a key amino acid residue in the binding of a direct DFXI to Factor Xa is Tyr-319 of SEQ ID NO:1. As is clear from the crystal data, the region between His-311 and Asp-320, extending over Tyr-319, is represented by a flexible loop. Alteration of this loop will dislocate Tyr-319 such that the binding of a direct Factor X inhibitor is impaired. Said impairment is to such extent that a recombinant Factor Xa protein, with an insertion in the region between His-311 and Asp-320, is less silenced by a DFXI, when compared to a protein without said insertion, and is still able to convert inactive prothrombin into the activeserine protease thrombin. Proof of concept for this model was provided by the applicant for three independent alterations of the region between His-311 and Asp-320, having a total of 21 amino acid residues (c-FX-A), 20 amino acid residues (c-FX-B), or 17 amino acid residues (c-FX-C), when compared to the 7 amino acids residues of the parental human FXa protein.

All three variant proteins disrupt binding to DFXIs without loss of their anti-coagulant function. On the basis of the experimental data, the technical solution of “polypeptide having an insertion of 1-20 amino acid residues” could be supported by the description.
The main points of the Chinese examiner were on two aspects. Firstly, the expression “corresponding to the region of amino acid residues between ...” is not definitive, as this indicates that there are other structures and species of coagulation FXas having different numbers of residues corresponding to His-311 and Asp-320 of SEQ ID NO:1, while the remaining amino acid sequences can be altered. Although the present application shows that there are conserved and altered amino acid residues corresponding to the region between Gly-289 and Asp-320 of SEQ ID NO: 1 between coagulation FX proteins of different species; the positions of such conserved amino acid residue will alter depending on the number of amino acid residues. However, the association between these conserved amino acid residues and DFXIs would not have been known to a person skilled in the art. Thus, it would have been difficult for a person skilled in the art to predict the insertion of amino acid residues between His-311 and Asp-320 regions of coagulation FXas for any structure and species having reduced sensitivity to inhibition of DFXIs while being able to restore hemostasis. Secondly, the expression "insertion of … amino acid residues", even if modified to be "insertion of 1-20 amino acid residues", still covers countless polypeptides with unknown functions, and these polypeptides cannot solve the specific technical problems. In cases where the relationship between "insertion of 1-20 amino acid residues in the region corresponding to His-311 and Asp-320 of SEQ ID NO: 1" and having reduced sensitivity to inhibition by DFXIs is not stated clearly, whether these polypeptides can solve the technical problem to be solved in the present application would have been unpredictable to a person skilled in the art. As such, claim 1 lacks support from the description.
However, this case has been granted with the technical solution of "insertion of 1 to 50 amino acid residues" in Europe, which is much wider than the protection scope of the currently pending claims in China.
[Case 2] Dispute on open-ended / close-ended definitions: PCT international application PCT/GB2009/000469, China National Phase application 200980144772.X and European phase application EP09784536A
Overview of China National Phase prosecution:
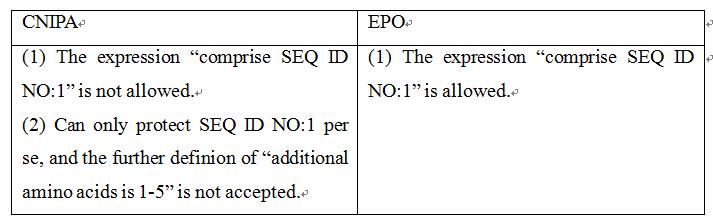
Claim 1 of the application as originally submitted is: “A method of isolating glycosaminoglycans capable of binding to a protein having a heparin-binding domain, the method comprising: (i) providing a solid support having polypeptide molecules adhered to the support, wherein the polypeptide comprises a heparin-binding domain; (ii) contacting the polypeptide molecules with a mixture comprising glycosaminoglycans such that polypeptide-glycosaminoglycan complexes are allowed to form; (iii) partitioning polypeptide-glycosaminoglycan complexes from the remainder of the mixture; (iv) dissociating glycosaminoglycans from the polypeptide-glycosaminoglycan complexes; (v) collecting the dissociated glycosaminoglycans.” The examiner, in the First Office Action, issued a rejection based on lack of support. However, the applicant held the technical solution without making amendments, and argued that on the basis of the sequences SEQ ID NOs:1-13 and 17 disclosed by the present application, in combination with the definition of “polypeptide comprises a heparin-binding domain”, those skilled in the art could expect the technical effects of the application. The examiner, in the Second Office Action, again held the rejection. According to this office action, claim 1 was amended to be “wherein the polypeptide molecules each comprise the amino acid sequence SEQ ID NO:1”. With regard to the amended claim 1, the examiner believes the use of “comprise” means amino acid residues of any amount and any type can be added, and the function of the sequence needs to be verified. Therefore, claim 1 lacks support from the description. When responding to the Third Office Action, the applicant amended “comprise” to be “consist of”, and further defined “wherein the number of such additional amino acids is 1-20”. On the basis of the amended claim 1, the examiner issued a Decision of Rejection, indicated that although the number of the additional amino acids was explicit, the amino acidssequence still changed relative to SEQ ID NO:1, and there existed a large number of amino acids in different forms. After changing the amino acid sequence, it should be verified through experiments whether the amino acid sequence still has the function of binding glycosaminoglycans. Later, the applicant amended the claim to be “wherein the number of such additional amino acids is 1-5” when requesting for re-examination. However, the panel held that although the number of the additional amino acids was at most 5, there still existed a large number of amino acids in different forms, and claim 1 still lacked support from the description. Finally, the case was granted with the sequence of SEQ ID NO:1 per se.
Overview of European Phase prosecution:
Claim 1 of the application as originally submitted was: “A method of isolating glycosaminoglycans capable of binding to a protein having a heparin-binding domain, the method comprising: (i) providing a solid support having polypeptide molecules adhered to the support, wherein the polypeptide comprises a heparin-binding domain; (ii) contacting the polypeptide molecules with a mixture comprising glycosaminoglycans such that polypeptide-glycosaminoglycan complexes are allowed to form; (iii) partitioning polypeptide-glycosaminoglycan complexes from the remainder of the mixture; (iv) dissociating glycosaminoglycans from the polypeptide-glycosaminoglycan complexes; (v) collecting the dissociated glycosaminoglycans.” That is, the claim was the same as claim 1 originally submitted in China. Later, the applicant submitted a modification, and defined “(i) providing a solid support having polypeptide moleculars that are, or comprise, SEQ ID NO:1…”, thus having a similar scope of protection to the claims submitted in response to the Second Office Action in China. The applicant then made other amendments to overcome other rejections, and the application was then granted with the aforementioned technical feature in claim 1.
Comparison between the prosecutions in China and in Europe:
Taking a look at the examination opinions made during the prosecution in China, it can be found that the applicant gradually narrowed the scope of the claims, and tried to prove from the mechanism that the technical solution of “wherein the number of such additional amino acids is 1-5” could be supported by the description. The applicant provided prior art documents to show that it is common general knowledge that a single turn of an α-helix requires 3.6 amino acid residues. In order to make a β-pleated sheet, two parallel chains of amino acids are required, requiring the chain to turn back on itself. As such, the additional 5 aminoacids and the N- and/or C- terminals could not be folded such that SEQ ID NO: 1 is covered, because it contains at most 5 amino acids at either end, and 3.6 of these would be required for the amino acid sequence to “turn” as an α-helix. Thus, there are simply not enough aminoacid residues in even a 5 amino acid sequence to allow the additional residues to fold and block the heparin binding domain, and so this sequence would not prevent the binding ofglycosaminoglycans to the heparin binding domain.
The main points of the Chinese examiner were on two aspects. Firstly, the defition with “comprise” was an open-ended definition, which means amino acid residues of any amount and any type could be added, and the function of the sequence needed to be verified. Secondly, once “additional amino acid” was defined, there existed a large number of amino acids in different forms, and those skilled in the art could not expect the technical effects of said different forms.
However, this case has been granted with the technical solution of “comprise SEQ ID NO:1” in Europe, which is much wider than the protection scope of the granted claims in China, and can only protect the sequence of SEQ ID NO:1 per se.
[Case 3] about the definition manner of homology / identity and fragment: PCT international application PCT/US2011/055932, China National Phase application 201180074173.2 and European phase application EP11873835A
Overview of China National Phase prosecution:
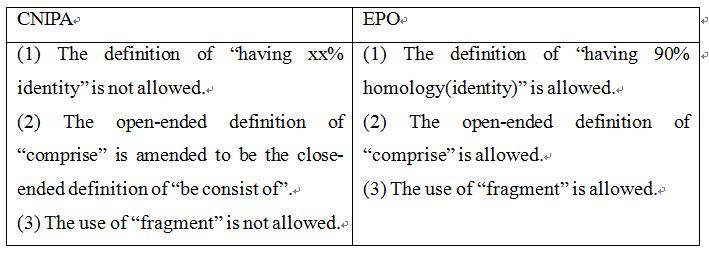
Claim 1 of the application as originally submitted was: "A nucleic acid molecule comprising a nucleotide sequence encoding for an HPV antigen selected from the group consisting of: SEQ ID NO: l; SEQ ID NO:3, SEQ ID NO:5, SEQ ID NO:7; fragments thereof having 90% homology; and combinations thereof”. The examiner, in the First Office Action, issued a rejection based on lack of support, indicating that: a nucleic acid or protein was defined by the expression "having at least 90 percent homology" and "fragment". Identity only represents a statistical value of sequence similarity, which is not directly related to the functions of the sequence. In addition, the open-ended definition manner of “comprise” was used to define a nucleic acid or protein, which means residues of any amount and any type can be added, and the function of the sequence needs to be verified. Therefore, the claim lacked support from the description. When responding to the First Office Action, the applicant amended “comprise” to be “consist of”, without amending the definition of "homology" and "fragment". The examiner, in the Second Office Action, again held the rejection. When responding to the Second Office Action, the applicant amended the expression of "having 90% homology" to be “SEQ ID NO:1; SEQ ID NO:3; fragments having at least 786 nt of SEQ ID NO:1 and fragments having at least 786 nt of SEQ ID NO:3”. With regard to the amended claim 1, the examiner stated that such a form of definition of fragments “having” a certain length includes cases where other sequences are arbitrarily added to the fragments of at least 786 nt. The addition of the sequences could change the spatial structure of the antigen. Whether the antigen could still retain the immunogenicity thereof and could be used as a vaccine needed to be verified by experimental data. Finally, the applicant amended said definitions to be “SEQ ID NO:1; SEQ ID NO:1 lacking the IgE leader sequence SEQ ID NO: 9” and obtained the patent right.
Overview of European Phase prosecution:
Claim 1 of the application as originally submitted was: “A nucleic acid molecule comprising a nucleotide sequence encoding for an HPV antigen selected from the group consisting of: SEQ ID NO: l; SEQ ID NO:3, SEQ ID NO:5, SEQ ID NO:7; fragments thereof having 90% homology; and combinations thereof.” That is, the claim is the same as claim 1 originally submitted in China. Later, the applicant submitted a modification, amending the term “homology” to be “identity” and further define the fragment with the expression “comprising at least 786 nucleotides of SEQ ID NO:1” , thus having a similar scope of protection to the claims submitted in response to the Second Office Action in China. Later, the applicant made modifications to overcome further inventiveness issues. The granted claim 1 in Europe was: “A nucleic acid molecule comprising a nucleotide sequence encoding a consensus fusion E6/E7 HPV antigen selected from the group consisting of: SEQ ID NO:1, fragments comprising at least 786 nucleotides of SEQ ID NO:1 and having at least 90% identity to SEQ ID NO:1 and wherein said fragments encode immunogens that enhance immune responses against HPV; and combinations thereof”. It can be seen that the expressions of “fragments comprising…” and “identity” were maintained.
Comparison between the prosecutions in China and in Europe:
Taking a look at the examination opinions made during the prosecution in China, it can be found that the applicant gradually narrowed the scope of the claims, and tried to obtain a wider scope as possible. In Chinese practice, defining a claim with the expressions of “fragments comprising…” and “having xx% of identity” is not allowed. However, in Europe, the open-ended definition of “comprise” and the use of “fragment” are allowed, and the definition of “having 90% of identity” (or even a littler lower than 90%) is generally acceptable, especially on the basis of the relevant experimental data being provided in the specification.
4、 Differences between China and Europe and Enlightenment obtained
As we all know, technicians in the field of biology can easily find technical solutions to avoid "a specific sequence". Taking DNA / RNA sequence as an example, due to the degeneracy of genetic codon, the new sequence obtained after degeneracy codon replacement of nucleotides at one or more sites can still retain the function of the original sequence. The same is true for the protein and polypeptide sequence. There are 20 natural amino acid substitution forms at each site of the protein sequence. Although a mutation in an important site of the functional domain may lead to the loss of function of the protein / polypeptide, the use of one or more amino acid substitution in more regions does not significantly affect the structure / function of the protein / polypeptide. Therefore, it is often not enough to protect the experimentally verified sequence itself.
From the above cases, it can be seen that in China, the open-ended definition of “comprise”, the use of “fragment”, and the definition of “having xx% of identity” are generally unacceptable. On the basis of Paragraph 4, Article 26 of the patent law of the People's Republic of China, the Evaluation Criteria for Supporting is clear: the scope disclosed in the specification is the contents that can solve the technical problems of the invention which can be reasonably summarized by those skilled in the art on the basis of the technical contents recorded in the specification and the teaching provided by the specification and in combination with the overall technical level in the technical field. However, in actual practice, there is still great subjectivity in what kind of generalization is "reasonable", and it is necessary to comprehensively consider the state of the art, the contribution of the present invention to the art, and the ability of those skilled in the art. Therefore, Chinese examiners often adopt a “strict” evaluation criterion for supporting issues. Although, in recent years, the scope of protect approved by the technical re-examination board in several litigation cases has been summarized on the basis of the embodiments to some extent, such as, the invalidation of glucoamylase patent No. ZL98813338.5, invalidation decision No. 17956: the final claim adopts the double definition of "99% or more homology" and "strain source", a large number of applications can only protect the specific sequences verified in the embodiment. As a result, the scope of protection is very narrow and easy to avoid, which will inevitably affect the economic value of the patent. In contrast, the European Patent Office allows the applicant to use the open-ended definition of “comprise”, the use of “fragment”, and the definition of “having xx% of identity” (90%, even as low as 80% when experimental data are provided in the specification), which undoubtedly can obtain a patent with a broader scope and great economic value.
As such, for Chinese applicants, when writing invention applications, it is necessary to make a full disclosure in the description, provide sufficient and representative experimental data, and summarize the technical solutions in the claims at multiple levels, so as to achieve the effect of "by charging you can attack, by fleeing you can defend" during the prosecution, and seek a suitable scope of protection for the invention. At the same time, even if the claim cannot be protected with a broader scope in China, it can still use the open-ended definition of “comprise”, the term of “fragment”, and the definition of “having xx% of identity”, so as to obtain a broader scope of protection in other countries or regions.
[References]
[1] Han Shiwei, Patent Agency, 2017, Issue 3, Pages 28-32.


Follow us






